Get Equipment
Get your own Ellab equipment to validate, qualify and monitor your processes.
Rent Equipment
Rent Ellab equipment for projects of any size and try our solutions before you commit.
Field Service and Consulting
Get on-site validation, qualification and calibration services as well as expert GMP consulting.
SteriSense® Components and Functionalities
Process Challenge Device (PCD)
The PCD has been specially designed to reflect the reference method originally developed by Dr. J. Bowie and J. Dick in the 1960’s. The function of the PCD is to “challenge” the steam penetration of a steam sterilizer in accordance with EN ISO 11140-4. Between tests, the PCD needs to be cooled down to ambient temperature (approx. 90 minutes), which is why it is a big advantage that the device can easily be disassembled from the body (logger) and be changed with a spare. Due to the PCD design, SteriSense is also able to check for the presence of non-condensable gases (NCG).
SteriSense® Triple Sensor
1. Temperature sensor that measures inside the PCD
2. Temperature sensor that measures the ambient temperature in the sterilization chamber
3. Pressure sensor that measures the ambient pressure value within the chamber
SteriSense® Data Logger
The SteriSense data logger contains a battery with enough capacity to run 1,000 test cycles of 30 min. The data logger itself is based on Ellab’s 3rd generation of the well-known TrackSense® Pro data loggers.

SteriSense® vs. Traditional Chemical Indicators
Unlike traditional chemical indicators, where you subjectively decide according to a change in color, SteriSense provides a much more in-depth verification of critical sterilization parameters. The SteriSense software also automatically generates and stores printable reports, ensuring electronic documentation of the process.
In summary, by using the SteriSense for full parametric release you gain the following advantages:
Economically efficient with high test volumes
Improved safety – objective results that eliminate the risk of false or grey zone readings
Time stamps included to increase reliability
Additional critical information – checks performed according to ISO 17665
Easy to store, retrieve and compare the data from a database – convenient and safe method that eliminates the need for guessing

User-friendly and Comprehensive Software
Absolute Compliance
SteriSense is extremely easy to use and requires only little training to operate. With just a single click in the software, the SteriSense measuring device will start to measure and record data. Once the Bowie Dick Test Program has ended, the device is simply placed back in the reader station where the software automatically processes the data and provides a test result.
All test results are saved electronically and can be presented in accessible reports with audit trails – simple and easy!
When compared to traditional methods, the SteriSense provides far more insight into critical sterilization parameters than previously possible. The standard report showcases all the results from the optional ‘checks’ performed by the software. When using the standard settings, a routine control test will be performed in accordance with EN ISO 17665.
SteriSense has been tested by a 3rd party certified test institute to comply with the reference method originally developed by Dr. J. Bowie and J. Dick, using a test procedure described in EN ISO 11140-4.
Easily identify the products suitable for your application with the Ellab Product Finder
Product Finder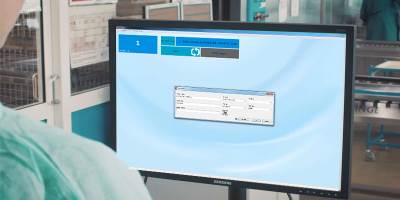
Step 1
Place the SteriSense measuring device in the reader station and open the SteriSense Software
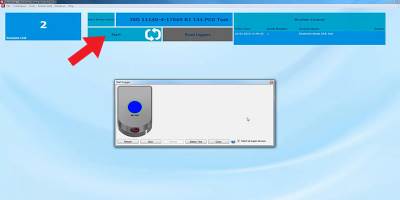
Step 2
Start the logger by clicking on “Start” and edit the sample number if necessary

Step 3
Place the SteriSense measuring device inside the steam sterilizer and start the Bowie Dick Test Program
Step 4
After the test program has completed, place the SteriSense back in the reader station to get the result
Analyzing Test Result and Generating Reports
Utilize the Full Potential of SteriSense® with ValSuite® Medical Software
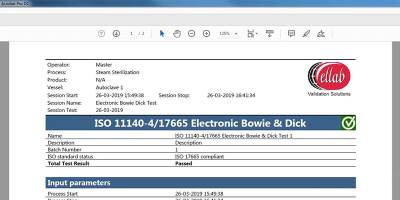
The result of the test will either pass or fail, which will appear on the screen shortly after the data has been processed.
The test results will be stored in the software and be included in an automatically generated PDF report.
When analyzing data, the SteriSense software can perform the following “checks”:
EN ISO 17665
- Phase detection (default)
- Holding time (default)
- Equilibration time (default)
- Maximum temperature deviation (default)
EN ISO 11140-4
- Air removal phase (optional)
- Heating phase (optional)
- Drying phase (optional)
Advanced Check
- Dilution factor calculation (optional)
You have the option of utilizing SteriSense to perform even more complex analysis than a simple routine Bowie Dick test control, like:
✔ Be a part of validation studies with Ellab TrackSense Data loggers
✔ Use for batch control during sterilization processes
✔ Perform a vacuum leak test
✔ Perform a check after the sterilizer has undergone service
Download our SteriSense® Brochure
Read more about our comprehensive solutions and services
