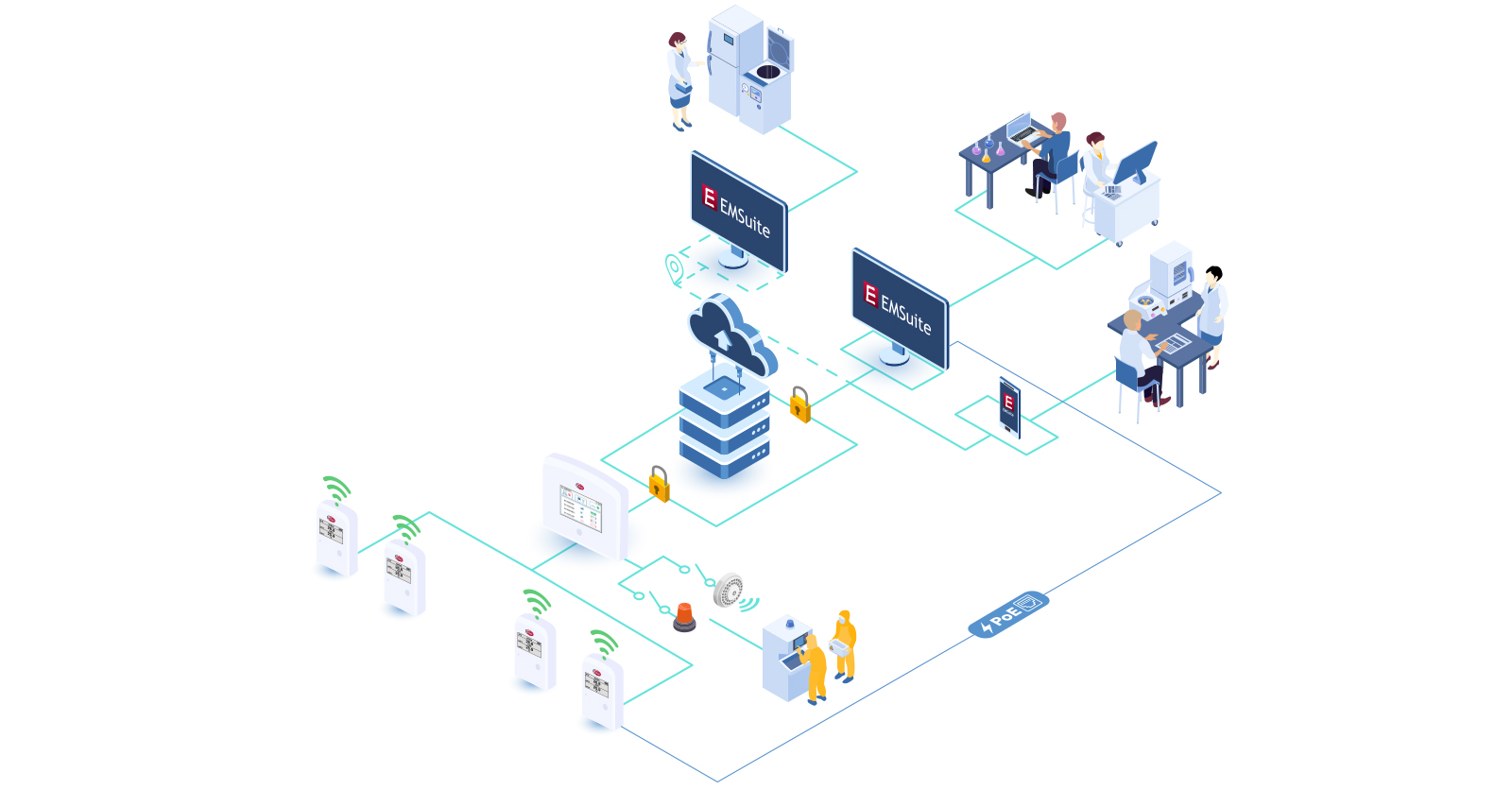
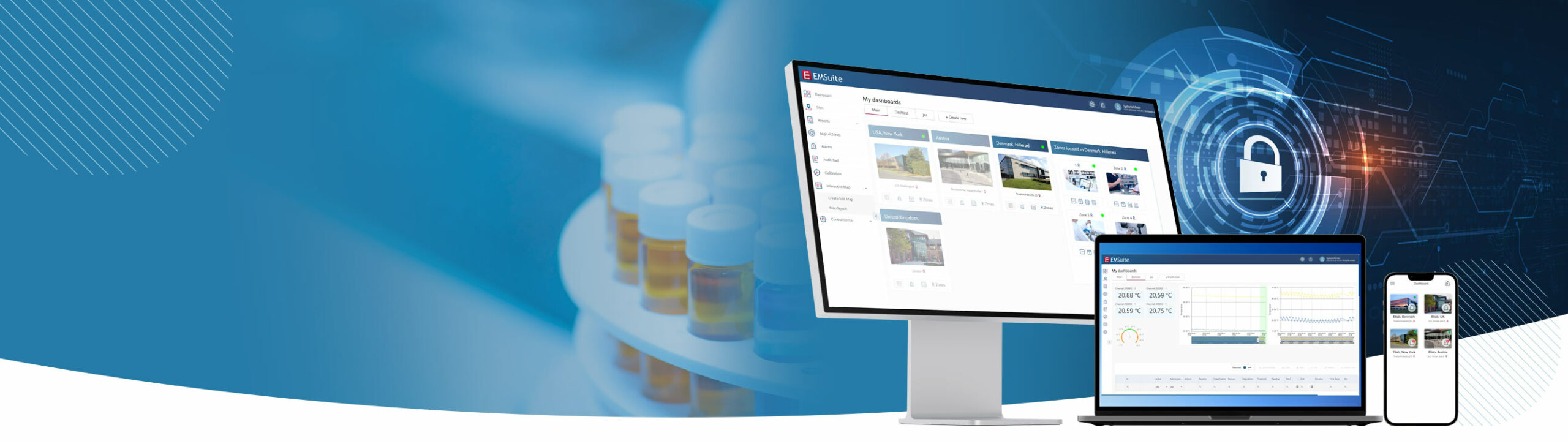
Environmental Monitoring
How to Use Compliance-Driven Checklists to Drive Success for Early Stage Biotech Companies

In the world of pharmaceutical and biotech research, following the rules is important for making sure your products meet quality standards.
For early-stage and R&D companies, understanding the significance of Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP) is vital. While GMP mainly applies to later stages, including clinical trials and product manufacturing, GLP is essential for non-clinical studies, particularly in toxicology and pharmacology. Integrating the principles of these practices early on can lay a sturdy foundation for future success and growth.